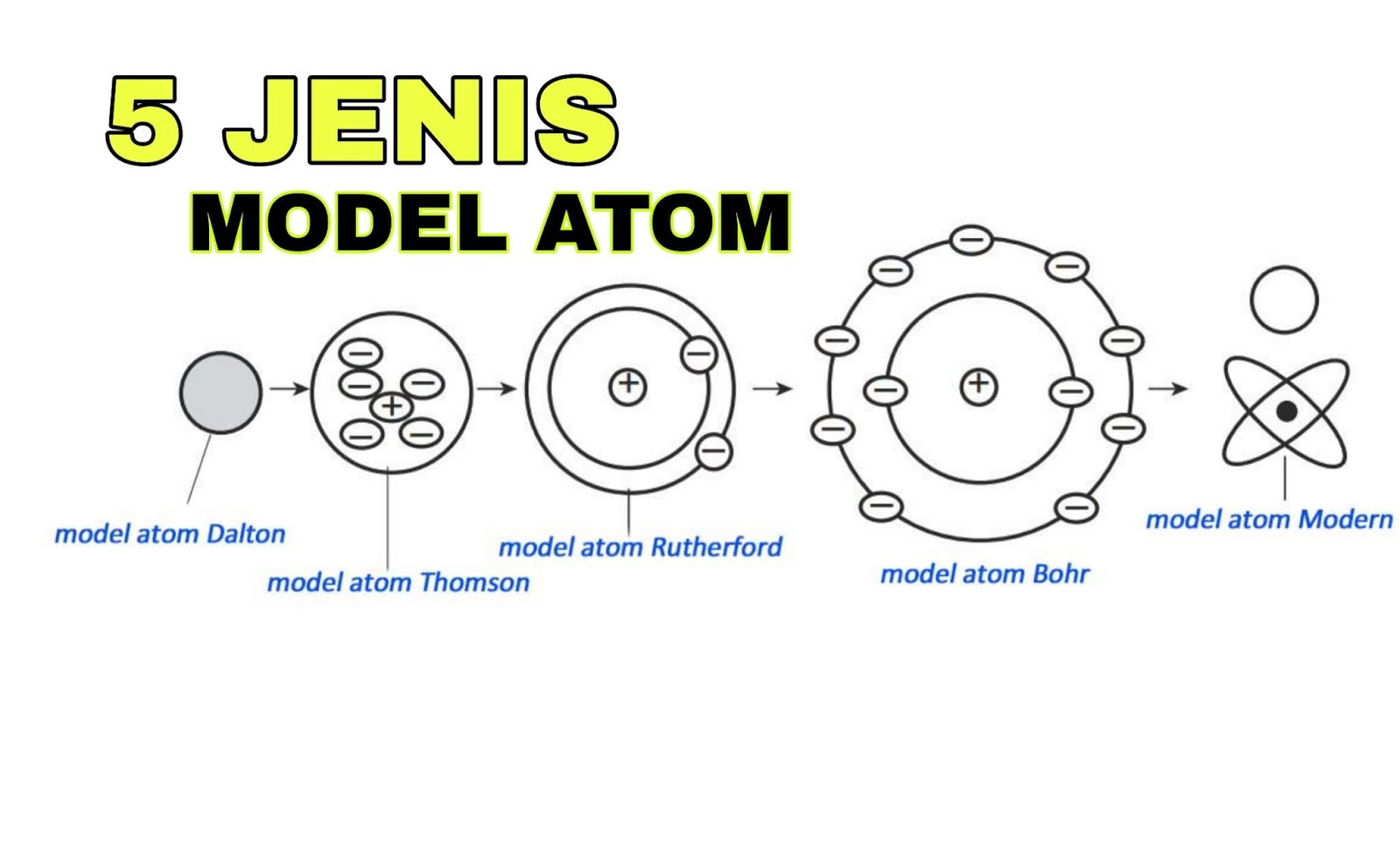
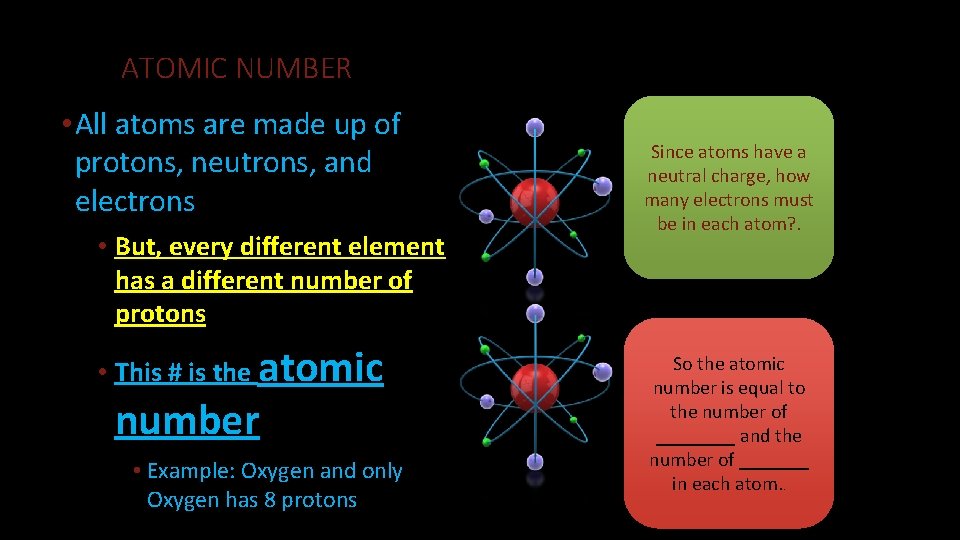
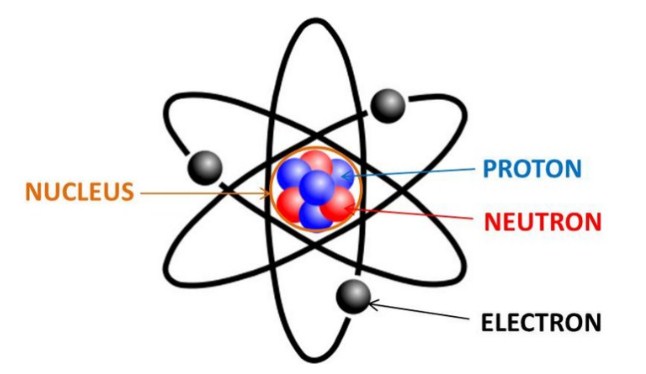
Dalton claimed that atoms of different elements vary in size and mass, and indeed this claim is the cardinal feature of his atomic theory. John Dalton and JJ Thompson proposed very different models of the atom. Both of them were of utmost importance in the development of future of the atomic model. John Dalton proposed that all matter is composed of very small things which he called atoms. This was not a completely new concept as the ancient Greeks (notably Democritus) had proposed that all matter is composed of small.
Atom John Dalton

John Dalton Atom

5 Parts Of Dalton's Atomic Theory
Democritus first suggested the existence of the atom but it took almost two millennia before the atom was placed on a solid foothold as a fundamental chemical object by John Dalton (1766-1844). Although two centuries old, Dalton's atomic theory remains valid in modern chemical thought.
Modern atomic theory is, of course, a little more involved than Dalton's theory but the essence of Dalton's theory remains valid. Today we know that atoms can be destroyed via nuclear reactions but not by chemical reactions. Also, there are different kinds of atoms (differing by their masses) within an element that are known as 'isotopes', but isotopes of an element have the same chemical properties. Many heretofore unexplained chemical phenomena were quickly explained by Dalton with his theory. Dalton's theory quickly became the theoretical foundation in chemistry. |
Back to index
Dalton's Theory Of The Atom
